Introduction: Introduction:
When the FDA closed down
Alpha Omega Labs on September 17, 2003, not only was
a extensive lab operation and nearly all its raw materials and
inventory destroyed, but so was the opportunity to protect
its most valuable asset of all: its reputation and trademarks --
the value of which had been carefully nurtured with life-saving
formulas for over a decade.

 We believe the
evidence is extensive and compelling that in the
aftermath of the destruction of our U.S. facility,
renegade forces lead by FDA agent, John Armand,
encouraged select parties to use our trademarks and
put out adulterated and misbranded product. The goal
was simple and straight-forward --- Cansema®, by that
time, had cured so many thousands of people of cancer
that a decision was made to allow other co-opted
entities to make different products using our
trademarks -- products pre-designed not to work. Our offices received
hundreds of complaint letters from Sept., 2003, to
March, 2007 (when our flagship company, Lumen Foods
[soybean.com] was sold). We forwarded these complaints to the
FDA. When the FDA finally
sent Toby a Warning Letter
in April, 2006 -- Toby did nothing for nearly two and
a half years, and then finally made
minor
bandaid alterations in August, 2008.
Ironically, the complaints in the FDA's Warning Letter
are virtually the same as those used to destroy
Alpha Omega Labs.
 No one can so blatantly
ignore "Warning Letters" from the FDA unless the agency never
intends to enforce them.
 The only way that
we can compensate individuals who have ordered "our"
products under fraudulent pretense -- all with
the knowledge and help of the FDA -- is to compensate
by making the real products available at a
dramatically reduced price. If the FDA were not a active
party in the theft of our intellectual property,
we would have legal remedies, but they have shown
themselves to only be helpful in ensuring that we
cannot protect our own U.S. trademarks.
How The Program Works
 The following table indicates
what products are available, and at what reduced price.
 A special Fake Product Compensation Program Order Form
has been created on our sister site. On this page you place your order, just
like you would on our regular
order page. It doesn't matter where in the world you are, as long as you meet the other requirements.
 This program now ends on December 31, 2009, and carries
two requirements:
- You must fill out Fake Product Questionnaire --
(which takes approximately 3 minutes). Included in this Questionnaire is a
statement by you that at the time of the alleged purchase of the fake product,
you believed you were purchasing the original formula. (We know you're out
there, because we've gotten hundreds of emails ourselves).
- You must email an invoice or other proof of purchase
as a file attachment to support@altcancer.net -- or
fax it, toll-free, to (888) 450-7909 -- or mail
your document(s) to the Miami address below. Note that to be eligible
for this program, your purchase must clearly represent a violation of one of
our trademarks: Cansema®, CanSupport (TM), or Alpha Omega Labs --- regardless
of how the words are spelled. They only have to be phonetically similar
such that the intent of the provider is to deceive the consumer.
- OPTIONAL: We are requesting -- but not making mandatory because availability
may be an issue -- that those taking part in this program send us what
is left of the original "fake product" to either our U.S. or Ecuadorean mailbox:
Herbologics, Ltd.
8345 NW 66th Street, #7093
Miami, FL 33166-2626
Herbologics, S.A.
Casilla 09-04-99 P
Guayaquil, Ecuador
- Then place your order.
 Critical to the destruction of AO Labs
in the United States was the coordinated involvement of my former associate,
Kevin Trudeau -- of late-night informercial fame -- and George Ackerson,
a former associate who bragged to associates about his role after my
imprisonment. (Additionally, the DOJ prosecutor in my case,
Larry Regan, identified George Ackerson to me in language so descriptive
that his identity was unmistakeable.)

 After my imprisonment in
September, 2003, Ackerson went about the business of setting up
imitation Alpha Omega Labs companies with two of our former
distributors -- first, in the form of
working with Toby McAdams to copy all of our materials; and then
working with Jennifer Wilson of Drake, Australia, to copy our
company name and all our materials. To this day, Jennifer Wilson
produces Cansema with the same name, same material -- even the
type style of Cansema (i.e. Cooper Black) is identical.
 To help identify the
source of the counterfeit materials she is producing with George Ackerson's
aid, I post her contact information below:
Jennifer Wilson
C.M.B., Drake NSW
2469 Australia
Phone: + 02 6737 6767 (mobile)
+ 61 4040150 671
|
|
Trademark /
Copyright Violators
 A partial list of companies
producing or promoting products covered under this Program includes:

 BloodrootProducts.com
BloodrootProducts.com -- Adulterated
and misbranded product; copyright and trademark
infringement, 2002-2008. Operates out of Montana.
 RisingSunProducts.com
RisingSunProducts.com -- Same entity as
BloodrootProducts.
 AltCancerCream.com
AltCancerCream.com -- Copyright and trademark
infringement. Owner / operators are Burt and Timothy
Hampton, 919 Highland, Magnolia, AR 71753.
 Jennifer Wilson
Jennifer Wilson (Australia) -- Wholesale copyright
and trademark infringement -- to the point where she claims
she is Alpha Omega Labs. (See below).
 George S. Ackerson
George S. Ackerson -- FDA informant who has
worked with and supplied stolen material from AO to
give to other entities -- including Jennifer Wilson
and Toby McAdams. His primary residence is
in Fort Benton, Montana.
 Health.Centreforce.com
Health.Centreforce.com -- Copyright and trademark
infringement.
 Elaine Hollingsworth /
Elaine Hollingsworth /
Troy Jones -- Promotes
health.centreforce.com and is producing a movie
using testimonials from Alpha Omega Labs' customers
with the intent of using the material to promote
health.centreforce.com. See
October, 2008 Ashwin.
|

 In Australia, the fake product
problem is even worse. FDA informant, George S. Ackerson -- after
bragging to friends in the aftermath of AO Lab's shutdown about
his critical involvement, worked with Jennifer Wilson in Drake, Australia,
to copy the company's materials, trademarks, copyrighted material,
and even our name -- Alpha Omega Labs . . .
See article in main column below.
 Fake Cansema:
Vegetable Oil,
Fake Cansema:
Vegetable Oil,
Flour, and Spice
 On August 24, 2004,
I had to report to federal court in Lafayette, Louisiana,
for my sentencing hearing. At the hearing I had two
witnesses who came to testify on my behalf. (This -- in
addition to the hundreds of testimonials we gave to my
attorney, Lewis Unglesby, of which he had given about
20 to the court). One of the two witnesses present was
a medical doctor with extensive experience in
the use of Cansema® (Edwards Smith, M.D. --
in fact, his own grandfather, also a medical doctor,
had privately used escharotics in the early 20th century
to treat cancers -- evidenced by formulas he left after
he passed on). On August 24, 2004,
I had to report to federal court in Lafayette, Louisiana,
for my sentencing hearing. At the hearing I had two
witnesses who came to testify on my behalf. (This -- in
addition to the hundreds of testimonials we gave to my
attorney, Lewis Unglesby, of which he had given about
20 to the court). One of the two witnesses present was
a medical doctor with extensive experience in
the use of Cansema® (Edwards Smith, M.D. --
in fact, his own grandfather, also a medical doctor,
had privately used escharotics in the early 20th century
to treat cancers -- evidenced by formulas he left after
he passed on).
 Before the hearing
-- a gut wrenching experience in which I was
sentenced to 33 months in prison -- we all sat down to
breakfast at a Lafayette restaurant. At that meeting,
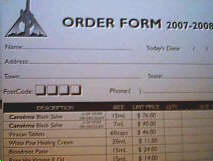
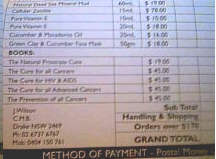
I was presented with the jar you see above -- the "Cansema" being
shipped by Toby McAdams (bloodroot products .com and risingsunhealth.com). Before the hearing
-- a gut wrenching experience in which I was
sentenced to 33 months in prison -- we all sat down to
breakfast at a Lafayette restaurant. At that meeting,
I was presented with the jar you see above -- the "Cansema" being
shipped by Toby McAdams (bloodroot products .com and risingsunhealth.com).
 I tasted the product for
authenticity. (I have formulated so many zinc-based escharotics
that on organoleptics alone I can identify the presence of
zinc chloride and one or more hydroquinone-based herbal
components). I tasted the product for
authenticity. (I have formulated so many zinc-based escharotics
that on organoleptics alone I can identify the presence of
zinc chloride and one or more hydroquinone-based herbal
components).
 I was shocked. I was shocked.
 The product was nothing
more vegetable oil, flour, and culinary spices. The product was nothing
more vegetable oil, flour, and culinary spices.
 Over the next four years,
the personnel at my one remaining manufacturing company
(Lumen Foods - soybean.com) wrote numerous letters and
made untold phone calls to officials all throughout the
FDA concerning this adulterated (i.e. contains ingredients
rendering it unfit for its intended purpose) and
misbranded (i.e. contains ingredients other than what is listed
on the label) -- an aggregious violation of
21 U.S.C.A. § 301 et seq. [1938]. Over the next four years,
the personnel at my one remaining manufacturing company
(Lumen Foods - soybean.com) wrote numerous letters and
made untold phone calls to officials all throughout the
FDA concerning this adulterated (i.e. contains ingredients
rendering it unfit for its intended purpose) and
misbranded (i.e. contains ingredients other than what is listed
on the label) -- an aggregious violation of
21 U.S.C.A. § 301 et seq. [1938].
 Above and beyond the
FDA issues was the consumer fraud. Specifically, many of the complaint
letters we received had to do with credit cards being charged
for product that was never shipped; non-response after repeated
attempts to get a proper refund; and attempts to avoid the
consumer after the sale. Above and beyond the
FDA issues was the consumer fraud. Specifically, many of the complaint
letters we received had to do with credit cards being charged
for product that was never shipped; non-response after repeated
attempts to get a proper refund; and attempts to avoid the
consumer after the sale.
 After years of seeking
a solution and then seeing that a "fig leaf Warning Letter"
sent out in April, 2006, was intended never to be enforced,
I and my staff came to the one inexorable conclusion -- namely,
that the FDA never intended to enforce the law as it pertained
to Toby. After years of seeking
a solution and then seeing that a "fig leaf Warning Letter"
sent out in April, 2006, was intended never to be enforced,
I and my staff came to the one inexorable conclusion -- namely,
that the FDA never intended to enforce the law as it pertained
to Toby.
 It is with this background
that after 6 years -- (we later learned that his illegal activities as it pertains to
Cansema actually began in the fall of 2002) -- that we realized
that unless this activity was made public and effort was made
on our behalf to compensate the victims, no proper resolution
would ever happen. It is with this background
that after 6 years -- (we later learned that his illegal activities as it pertains to
Cansema actually began in the fall of 2002) -- that we realized
that unless this activity was made public and effort was made
on our behalf to compensate the victims, no proper resolution
would ever happen.
|